Hello, welcome to Hangzhou Zhengda Medical Co., Ltd.
NEWS CENTER
PRODUCTS CLASS
-
New Registration Certificate Issued: Hangzhou Zhengda Medical's Automatic Tourniquet System More Clinically Adaptive2025-9-4 hits:70Hangzhou Zhengda Medical Equipment Co., Ltd.' automatic tourniquet system (models YTQ-G & YTQ-H) is newly registered and compliant with latest regulations. Featuring dual-channel independent control, IVRA function, smart LOP , automatic pressure calibration, it provides safe, reliable, and high-precision tourniquet solutions for orthopedic and surgical procedures.
-
Hangzhou Zhengda Medical engineers went to a hospital in Guangzhou to conduct special training on lower limb joint rehabilitation device CPM-E2025-8-29 hits:250Hangzhou Zhenda Medical engineers held CPM-E lower limb rehabilitation device training at a Guangzhou hospital. The session showed how the device supports post-surgery recovery for hip, knee, and ankle joints. With adjustable angles, overload protection, and easy operation, the CPM-E helps medical staff provide safe and effective rehabilitation for patients.
-
Hangzhou Zhengda Medical's Touch Screen Automatic Tourniquet System Successfully Obtained Brazilian Medical Device Registration Certificate2025-8-15 hits:632Hangzhou Zhengda Medicals touchscreen automatic tourniquet system gained ANVISA medical device registration in Brazil, meeting strict safety and compliance standards.
-
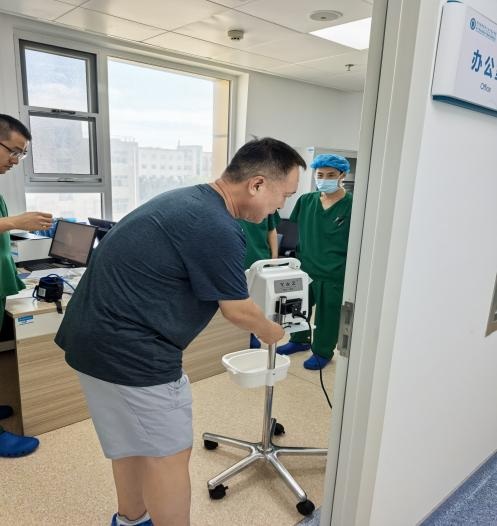
Hangzhou Zhengda Medical engineers demonstrated the precise use of the automatic tourniquet system2025-8-13 hits:641Hangzhou Zhengda Medical conducted hands-on training at a Shandong hospital, demonstrating the precise use of its automatic tourniquet system. The session covered operating principles, touchscreen features, dual pressure display, IVRA function, and clinical application, helping medical staff improve surgical safety and efficiency.